Dr. Lu’s research involves the use of nanoparticle-based formulations to deliver therapeutic and diagnostic agents. These are primarily applied in the diagnosis and treatment of cancer.
1. Neutron-Activatable Nanoparticles for Targeted Radionuclide Therapy of Tumors
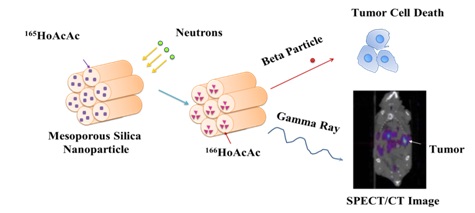
The goal of this project is to develop a neutron-activatable biologically targeted radiotherapeutic nanoparticle for the treatment of peritoneal metastases in ovarian cancer, the main cause of morbidity and mortality of this disease.
We hypothesize that administering these NPs intraperitoneally can deliver efficacious radiation doses specifically to ovarian tumors. The novelty of this agent is that it is produced by the neutron activation of stable Holmium atoms ( 165Ho) in the nanoparticle matrix. The radionuclide produced by neutron irradiation of these NPs, 166Ho, decays by the emission of beta particles capable of delivering sufficient absorbed radiation doses to targeted cells. The surface of these nanoparticles can be functionalized targeting ligand directed against ovarian tumors. Retention of the particles in the peritoneal cavity can be determined using SPECT/CT imaging as 166Ho also emits 81 keV photons.
The process of rendering the nanoparticles radioactive after they have been prepared allows their preparation in compliance with FDA current Good Manufacturing Practices without having to handle hazardous quantities of radioactivity, and permits flexibility in terms of adding different targeting ligands.
Hargrove D, Liang B, Kashfi-Sadabad R, Joshi G, Gonzalez-Fajardo L, Glass S, Jay M, Salner A, Lu X*. Tumor-mesoporous silica nanoparticle interactions following intraperitoneal delivery for targeting peritoneal metastasis. Journal of Controlled Release. 2020, 328:846-858.
Di Pasqua AJ, Yuan H, Chung Y, Kim JK, Huckle JE, Li C, Sadgrove M, Tran TH, Jay M, Lu X*. Neutron-activatable holmium-containing mesoporous silica nanoparticles as a potential radionuclide therapeutic agent for ovarian cancer. Journal of Nuclear Medicine. 2013, 54:111-116
Di Pasqua AJ, Huckle JE, Kim JK, Chung Y, Wang AZ, Jay M and Lu X*. Preparation of neutron activatable holmium nanoparticles for the treatment of ovarian cancer metastases. Small. 2012, 8 (7): 997-1000
2. Self-Assembled Brush Block Copolymer-Nanoparticles For Augmented Tumor Delivery
Nanotechnology-based chemotherapeutics have exhibited clear benefits when compared with unformulated drugs, including reduced patient side effects, improved circulatory and tumor retention and greater targeting efficiency by passive (leaky tumor vasculature) or active (use of targeting ligands) mechanisms. The rational design of nanoparticles plays a critical role since their structural and physical characteristics, such as size, charge, shape, and surface properties determine the biodistribution, pharmacokinetics, internalization and safety of the drugs.
We aim to establish modular polymer based nanocarrier platforms for effective delivery of potent chemotherapeutic agents based upon precise understanding of the relationship between physical characteristics of polymer nanocarriers and efficacy and safety of delivered drugs, which is currently not highly advanced.
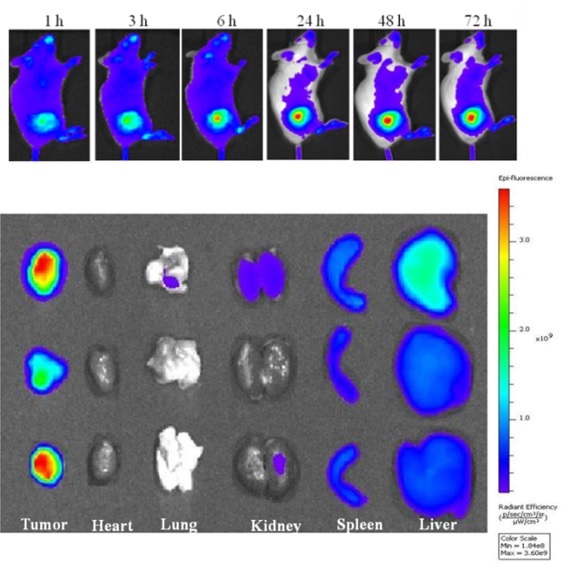
In collaboration with Prof. Rajeswari Kasi in the Institute of Materials Science at UConn, we have prepared self-assembled nanoparticles from two new libraries of amphiphilic cholesterol-based brush-like block copolymers: (1) Polynorbonene bearing a cholesterol block and a poly(ethylene glycol) (PEG) block (PNBCh9-b-PNBPEG)) and (2) polymethacrylate bearing a cholesterol block and a polyethylene glycol block with biocleavable/reducible disulfide bonds (PC5MA-SS-PEO). Initial in vitro and in vivo studies using tumor-bearing mice have shown the effective tumor accumulation of doxorubicin-loaded nanoparticles and enhanced nucleus uptake of doxorubicin for cytotoxicity to cancer cells.
Perry JM, Tao F, Roy A, Lin T, He XC, Chen S, Lu X, Nemechek J, Ruan L … & Li L. Overcoming Wnt–β-catenin dependent anticancer therapy resistance in leukaemia stem cells. Nature Cell Biology. 2020, 1-12.
Gonzalez-Fajardo L, Mahajan LH, Ndaya D, Hargrove D, Manautou JE, Liang BT, Chen MH, Kasi RM, and Lu X*. Reduced In Vivo Toxicity of Doxorubicin by Encapsulation in Cholesterol-Containing Self-Assembled Nanoparticles. Pharmacological research. 2016, 107 :93-101
Nguyen CT, Tran TH, Lu X*, Kasi RM*. Redox-Sensitive Nanoparticles from Amphiphilic Cholesterol-Based Block Copolymers for Enhanced Tumor Intracellular Release of Doxorubicin. Nanomedicine: Nanotechnology, Biology, and Medicine. 2015, 11(8): 2071–2082
Tran TH, Nguyen CT, Gonzalez-Fajardo L, Hargrove D, Song D, Deshmukh P, Mahajan L, Ndaya D, Lai L, Kasi RM, and Lu X*. Long Circulating Self-Assembled Nanoparticles from Cholesterol-Containing Brush-Like Block Copolymers for Improved Drug Delivery to Tumors. Biomacromolecules. 2014, 15 (11): 4363–4375
Nguyen CT, Tran TH, Lu X, Kasi RM. Self-assembled nanoparticles from thiol functionalized liquid crystalline brush block copolymers for dual encapsulation of doxorubicin and gold nanoparticles. Polymer Chemistry. 2014, 5: 2774-2783
3. Quercetin Nanoemulsion Tablets for Enhancing its Oral Bioavailability and Chemoprotective Effect
This project was supported by the Diet and Health Initiative and represents an innovative area of research at UCONN by multi-disciplinary investigators from pharmaceutical sciences /nanotechnology, nutritional biochemistry, and cancer biology.
Quercetin is a dietary polyphenol that has chemoprotective activities. However, its oral bioavailability is very low. The goal of this project is to develop an advanced phytochemical delivery system for quercetin that enhances its oral bioavailability and improves the effectiveness of chemotherapy. This will be achieved using an optimal design matrix to determine the composition of a quercetin self-emulsifying drug delivery system (SEDDS) that is stable and yields the greatest bioavailability after oral administration to rats, and by demonstrating the ability of these formulations to potentiate the effects of the chemotherapeutic agent paclitaxel in retarding tumor growth in a mouse xenograft tumor model.
Innovations include the oral administration of a nanoemulsion containing a bioactive food component that has the potential to improve the therapeutic index of standard chemotherapeutic agents, and the nanoemulsion manufacturing methods that are readily scalable, reproducible and potentially compliant with FDA current Good Manufacturing Practices.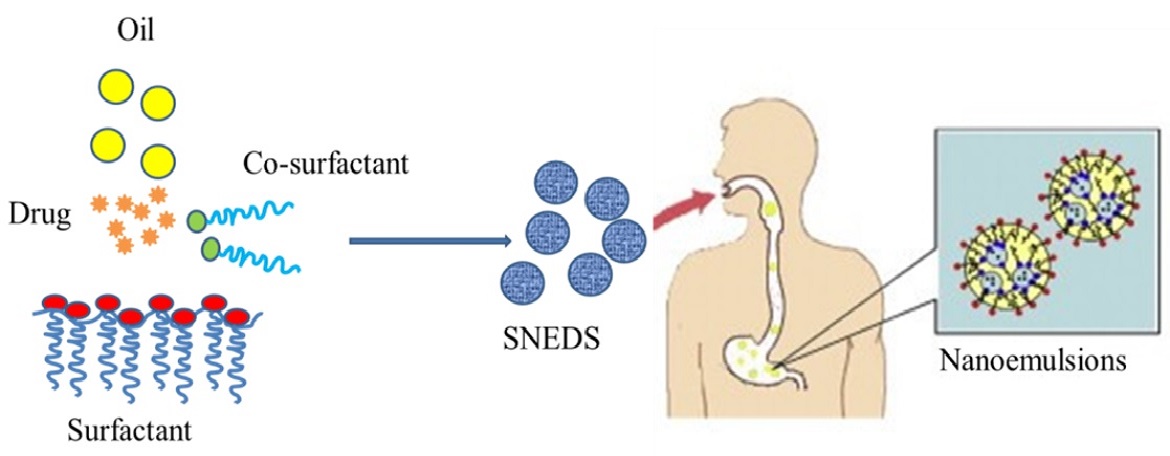
Tran TH, Guo Y, Song D, Bruno R, Lu X*. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103 (3): 840-852.
However, products prepared with nano-emulsifying technology display inherent shortcomings due to their liquid nature, such as high-production costs, relative low drug stability and limited shelf-life.
With the support of the Center for Pharmaceutical Processing Research (CPPR) and in collaboration with Dr. Chaudhuri’s group (School of Pharmacy/UConn), Lu Lab looked into the manufacturability of solid SEDDS (S-SEDDS) with the ultimate goal of improving product performance and stability. Our group has focused mainly on the preparation of S-SEDDS tablets. The tendency of liquid SEDDS to compromise the physical integrity and mechanical strength of conventional tablets is a major concern from a manufacturing perspective. Our group addressed such concerns by thoroughly and systematically investigating the impact of commonly used porous excipients on the manufacturability and product performance of S-SEDDS tablets.
Beringhs AO, Minatovicz BC, Zhang GZ, Chaudhuri B and Lu X*. Impact of Porous Excipients on the Manufacturability and Product Performance of Solid Self-Emulsifying Drug Delivery Systems.AAPS PharmSciTech. First Online: 14 September 2018. doi: 10.1208/s12249-018-1178-x
4. Impact of Freeze Drying Process on Stability of Nanocarriers
Freeze-drying is an effective approach to improve the long-term stability of nanomedicines. Lyoprotectants are generally considered as requisite excipients to ensure that the quality of nanoparticles is maintained throughout the freeze-drying process. However, depending on the type of nanoparticles, the needs for lyoprotectants or the challenges they face during freeze-drying may be different. We compared and identified the impact of freeze-drying on key characteristics of three types of nanoparticles: solid lipid nanoparticles (SLNs), polymeric nanoparticles (PNs), and liposomes. Sucrose, trehalose, and mannitol were added to nanoparticle suspensions before freeze-drying. The same conservative freeze-drying conditions with controlled ice nucleation at −8°C were employed for all formulations. The collapse temperatures of nanoparticle formulations were found to be the same as those of the lyoprotectant added, except PN formulation. Likely the poly(vinyl alcohol) (PVA) in the formulation induced a higher collapse temperature and retardation of drying of PNs. Freeze-drying of both SLNs and liposomes without lyoprotectants increased particle size and polydispersity, which was resolved by adding amorphous disaccharides. Regardless of the addition of lyoprotectants, freeze-drying did not alter the size of PNs possibly due to the protection from PVA. However, lyoprotectants were still necessary to shorten the reconstitution time and reduce the residual moisture. In conclusion, different types of nanoparticles face distinct challenges for freeze-drying, and lyoprotectants differentially affect various stability and quality attributes of freeze-dried nanoparticles.
5. In vitro and in vivo Assessment of Ophthalmic Ointments for Generic Product Equivalence
The main purpose of this study is to evaluate qualitative (Q1) and quantitative (Q2) equivalent oleaginous ophthalmic ointments of tobramycin (TOB) and dexamethasone with different physicochemical properties and identify critical process/quality attributes using various in vitro methods of characterization. We found that source and composition of the petrolatum played a more critical role in determining the rheological properties compared to the method of preparation. Results demonstrated the impact of the source of TOB, excipients and manufacturing differences on the quality attributes of TOB ophthalmic ointments.
Patere S, Newman B, Wang Y, Choi S, Mekjaruskula C, Jay M and Lu X*. Influence of In vitro Release Methods on Assessment of Tobramycin Ophthalmic Ointments. International Journal of Pharmaceutics. 2020, 590, 30:19938
Patere S, Newman B, Wang Y, Choi S, Vora S, Ma A, Jay M, and Lu X*. Influence of Manufacturing Process Variables on the Properties of Ophthalmic Ointments of Tobramycin. Pharmaceutical Research. 2018, 35:179.
6. Bioresponsive Theranostic Nanovectors for Improving Drug Therapeutic Ratios
Many studies now suggest that physiologic barriers to drug delivery to tumors represent a major mechanism of drug resistance to chemotherapeutic agents. Physiologic abnormalities in tumors include increased interstitial fluid volume and pressure, decreased oncotic gradients and increased interstitial deposition of high molecular weight proteins such as collagen. All of these factors decrease convectional delivery of molecules and nanoparticles into tumor interstitial space and to tumor cells therein.
Previous pre-clinical studies and clinical trials have demonstrated that dexamethasone (DEX) administered prior to chemotherapy increases the effectiveness of chemotherapy and decreases toxicity. We hypothesize that DEX exerts these effects by reducing these physiological barriers through various mechanisms such as those mediated by cytokine release and protein deposition. However, systemic DEX administration induces a number of toxicities which significantly limit its clinical application.
The overall goal of this project is to deliver DEX specifically to tumors using a bioresponsive triggered-release mechanism in order to minimize systemic toxicity and enhance the uptake of cancer therapeutics into tumors. This will be achieved using a pegylated nanoparticle drug delivery system containing dexamethasone-palmitate (DEXP) in which the release of DEX in tumor regions will be triggered by esterases.
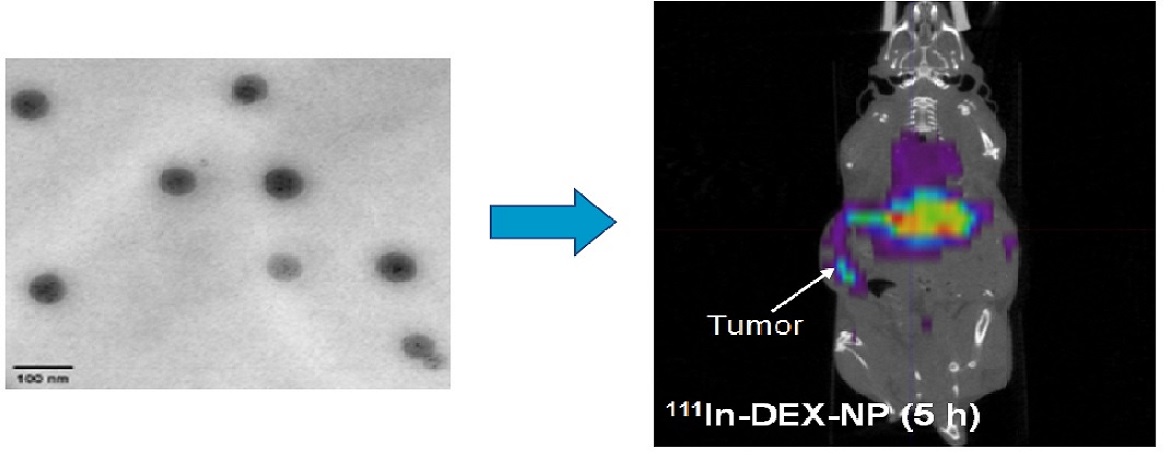
Studies in animal models which suggest that failure of drug delivery to the tumor interstitial space is a major mechanism of drug resistance have not been confirmed in patients with cancer. Tissue pharmacokinetics in patients is not yet practical in either clinical research or practice settings. To overcome this limitation, we are preparing dual therapeutic/imaging nanovectors by entrapping DEXP in nanoparticles that also have 111In bound to their surfaces. These nanoparticles will allow for the examination of this important hypothesis in clinical medicine by single photon emission computed tomography (SPECT). Noninvasive imaging of nanoparticle uptake in tumors may be used to calculate patient-specific doses of chemotherapy and move us closer to the goal of true “personalized medicine”
Kim JK, Yuan H, Nie J, Yang YT, Leggas M, Potter PM, Rinehart J, Jay M and Lu X*. High payload dual therapeutic-imaging nanocarriers for triggered tumor delivery. Small. 2012, 8: 2895-2903
Kim JK, Howard MD, Dziubla TD, Rinehart JJ, Jay M and Lu X*. Uniformity of drug payload and its effect on stability of solid lipid nanoparticles containing an ester prodrug. ACS Nano. 2011, 5 (1): 209-216